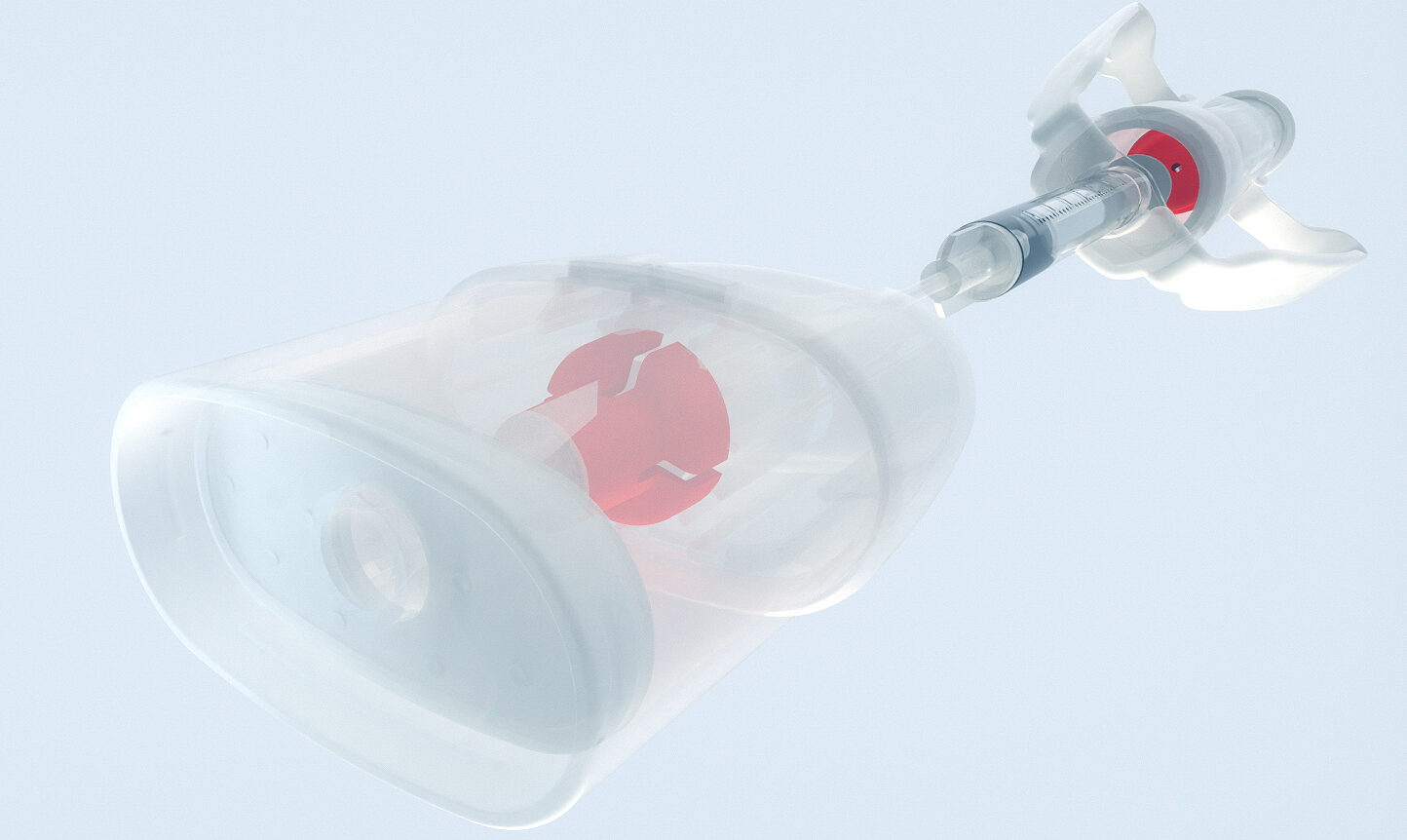
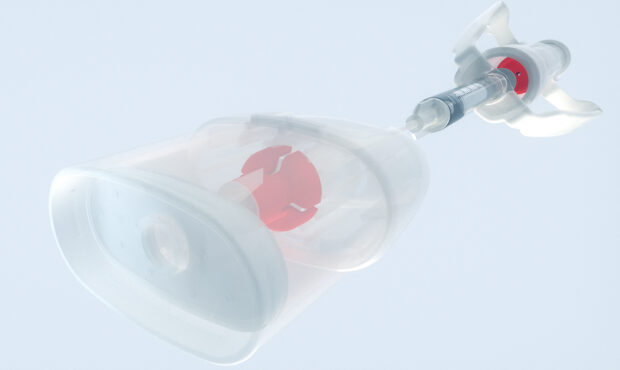
Successful trial phase for treatment of COVID-19 induced hypoxemia Phase IIb clinical trial executed with Medspray nozzle technology for treatment of COVID-19 induced hypoxemia.
Our daughter company Resyca has supplied Pulmospray soft mist inhalation devices for a therapy for the treatment of COVID-19 induced hypoxemia with low molecular weight Heparin. The outcome of this study indicates that it is feasible to nebulize Heparin using the Medspray nozzle technology. This means that our devices can be used in the treatment of severe acute respiratory distress syndrome for hospitalized patients affected by COVID-19. The full results of the study were recently published in the MDPI journal and can be found here.
The improvement was particularly striking in cases with severe hypoxemia. During the 10-day treatment period, low molecular weight Heparin was shown to significantly improve breathing capabilities when
delivered via soft mist inhaler, compared to the control group who received standard care.
In the device group, 45.7% (16/35) of patients improved one degree of severity, 34.3% (12/35) of the patients improved two degrees, and 8.6% (3/35) improved three degrees. The control group's results
were more heterogeneous: 35% (14/40) improved one degree of severity, 2 patients (5%) improved two degrees, and only 1 patient (2.5%) improved three degrees. The hypoxemic issues of a majority of the patients in the control group did not improve after standard treatment; three patients in the control group even needed to be intubated sometime during the 10-day period. For more information regarding the Soft Mist Inhalation device, the nozzle technology or the clinical study, feel free to contact Medspray or Resyca.