Medspray Pharma BV has secured the second round of funding from the Bill & Melinda Gates Foundation to develop a non-invasive liquid surfactant nebulizer for premature babies. We aim to develop a method that can be used on newborns worldwide; regardless of the locally available levels of medical resources. Thanks to the development agreement, Medspray can continue its work on the non-invasive liquid surfactant nebulizer.
Medspray started developing its liquid surfactant nebulizer in 2021, through an initial funding round supported by the Bill & Melinda Gates Foundation. This year, the work resulted in the first prototypes. These prototypes are now being tested at the Seattle Children’s Hospital to validate the nebulization technique for liquid surfactants.
Taking the next step with a second round of funding
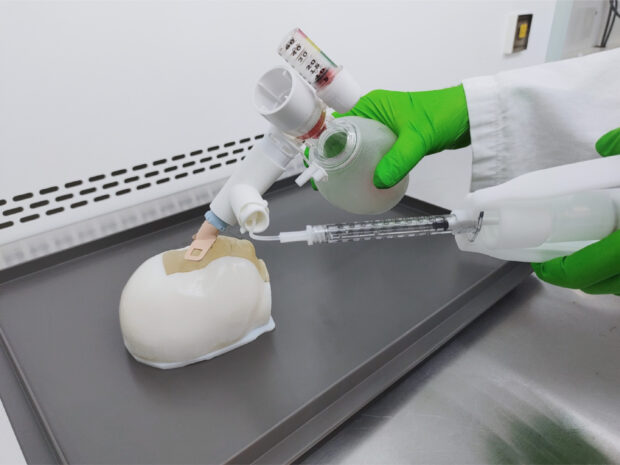
As a continuation of the previous grant, the second round of funding, is intended to optimize the aerosolization device. This device can be used for the delivery of liquid surfactants to the lungs of premature infants with newborn respiratory distress syndrome (NRDS).
The delivery device has the unique quality that it can be used inside and outside health care facilities in low- and middle-income countries, thanks to the manual resuscitation or ventilation bag.
Collaborations with neonatal care experts
On top of the grant continuation, we are proud and excited to have teamed up with key opinion leaders in neonatal care in the US. We also work with a team from the foundation focused on maternal and newborn health. Together, we aim to get the best results within the span of the agreement.
Want to know more about what we do and about this project?
You can keep track of our progress through our social media channels: follow us for more exciting news and updates on our project supported by grant funding from the Bill & Melinda Gates Foundation.